Model Turbines
Model Turbines
Home › Forums › Stationary engines › Model Turbines
- This topic has 598 replies, 28 voices, and was last updated 31 March 2025 at 14:47 by
Turbine Guy.
-
AuthorPosts
-
28 February 2019 at 17:19 #398262
Turbine Guy
Participant@turbineguyI would like to clarify part of the information in the 27/2/2019 post. I stated that the maximum rotor velocity coefficient for widely spaced blades was approximately 0.90 and for closely spaced blades approximately 0.74. This is misleading. The velocity coefficient was largest in the wide blade spacing because all the flow went into one blade channel. The best case tested for the narrow blade spacing was 0.74 but that was partially due to the nozzle overlapping the blades. With this condition, a portion of the flow was going into empty blade channels on each side and suddenly expanding. This was why Dr. Balje stated the admission length (a) should be 1, 2, 3, and etc. times the pitch (t). His testing indicated that for blade channels with full flow the maximum rotor velocity coefficient was approximately 0.90. As I stated in my 18/2/2019 post, keeping the blades full was an important part of using 4 velocity stages.
Following up on the post of 27/2/2019, the test that I ran using my airbrush compressor to run the turbine had the following parameters. The air flow was 1.74 lbm/hr, the inlet pressure was 24 psig, and the exhaust pressure was assumed to be approximately 0 psig. The turbine had an EP2508 propeller attached as shown in the picture below. The maximum speed obtained in this test was 17,012 rpm. For the EP2508 propeller I was using, the power required to turn at that speed is approximately 1.92 watts based on an online calculator. This matched with the amount of power used by an electric motor that ran the same propeller at approximately the same speed and the motor efficiency was given for that speed. I thought the simplest way to improve the performance was to increase the speed. I first looked at finding a smaller propeller with given performance data. I could only find a couple of propellers that gave the power required to turn at given speeds and they were too small. I then looked at using a speed reducer. I calculated that if I used the existing turbine and the EP2508 propeller combined with a 2.5:1 ratio speed reducer I would be able to spin the propeller at about 21,600 rpm. The corresponding power required by the propeller at this speed would be approximately 3.96 watts. For this propeller speed, the turbine speed would be 54,000 rpm. The only speed reducers I could find used spur gears and were intended to have input speeds of around 20,000 rpm. The dental turbines that run at speeds in the 200,000 rpm range use friction drive speed reducers. Making a friction drive speed reducer could be an option since power increase would be substantial.
 1 March 2019 at 15:54 #398345
1 March 2019 at 15:54 #398345Turbine Guy
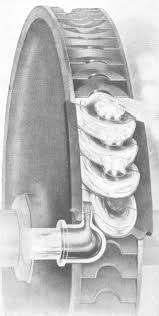
Participant@turbineguyAnother way to improve the performance is using velocity staging. The information I found for a Terry turbine that I added in the post of 18/2/2019 showed that turbine had an efficiency of approximately 48% using 4 velocity stages. The Terry turbine’s tangential flow makes velocity staging relatively easy. The reversing chamber is the most complicated part. The flow exiting the rotor blades flows into similar blades in the reversing chamber. The blades in the reversing chamber need to reverse the flow direction and the flow exiting the reversing chamber needs to flow into the next pair of blades in the rotor. Reversing and advancing the flow correctly is the most difficult part. I noticed in pictures of Terry turbine reversing chambers the blade spacing appeared to be about equal to the length of admission of the nozzle. Also the blade spacing in the reversing chamber and the rotor appeared to be about the same. I failed in my attempts to accomplish this with the ideal blade width of 3.25 times the nozzle diameter recommended by Dr. Balje. I scaled one of the pictures I have for a Terry turbine reversing chamber and the blade width appeared to be about 6 times the nozzle diameter. Increasing the blade width allowed me to come up with the design shown in the drawing below. Increasing the blade width increases the length of travel of the flow and consequently reduces the rotor velocity coefficient. Based on the efficiency of the example Terry turbine, the loss for increasing the blade width must not be too high. Because the spouting velocity of the nozzle using my airbrush compressor is about half the spouting velocity of the example Terry turbine, I decided to use two velocity stages.
 My preliminary analysis of this design indicates the two velocity stages will be about right and I should be able get over 4 watts of power using the EP2508 propeller. I will get into more details on this model in my next post.4 March 2019 at 17:27 #398682
My preliminary analysis of this design indicates the two velocity stages will be about right and I should be able get over 4 watts of power using the EP2508 propeller. I will get into more details on this model in my next post.4 March 2019 at 17:27 #398682Turbine Guy
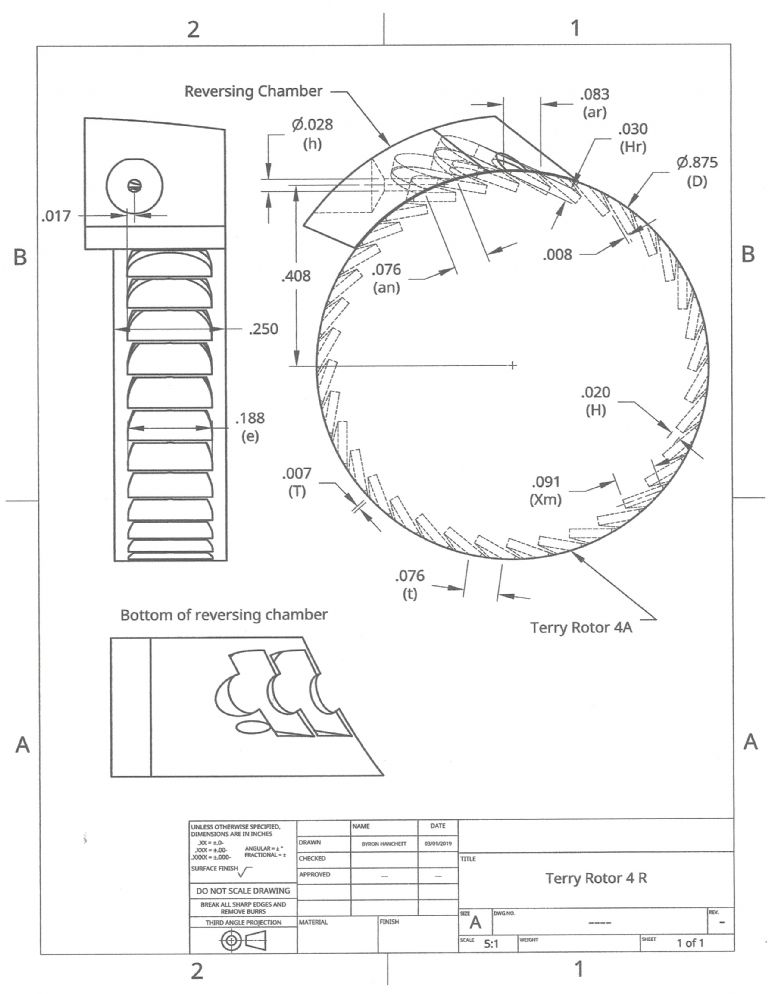
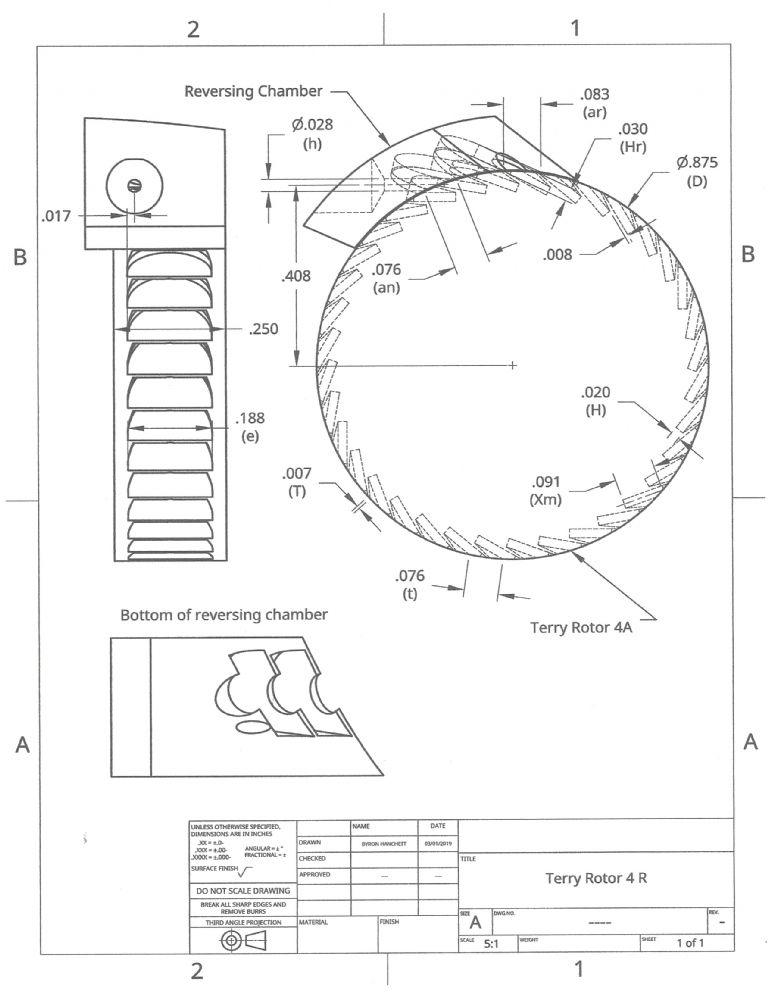
Participant@turbineguyThe attached drawing is a revised version of the drawing sent in the last post. To try to optimize the flow paths, I increased the number of blades in the rotor and will need to cut the blades in two steps instead of one. Increasing the number of blades required the keyseat cutter to be different for the reversing chamber and the rotor. Both keyseat cutters are 3/16 in. diameter but the width (H) of the rotor cutter is 0.20 in. and the width (Hr) of the reversing chamber cutter is 0.30 in. Cutting the blades in two steps instead of one reduced the blade blockage length (T) to 0.007 in. The previous version had a blockage length of 0.042 in. Using two different keyseat cutters and doubling the number of steps to cut the blades increases the cost and machining time considerably, but I think is worthwhile. The blade pitch (t), the nozzle admission length (an), and the reversing chamber admission length (ar) are 0.076 in., 0.76 in., and 0.83 in respectively. Having them close to the same value helps the filling of the blades. My calculations estimated that I could get over 4 watts with this configuration based on the values of rotor velocity coefficients Dr. Balje found in the testing of Terry turbine rotors. Since the blades of the reversing chamber are similar to the blades of the rotor, I calculated the reversing chamber velocity coefficients in a similar way.
5 March 2019 at 18:54 #398842Turbine Guy
Participant@turbineguyMy airbrush compressor has an output of approximately 1.74 lb/hr of air at a pressure of 24 psig. Assuming close to zero back pressure the amount of energy is approximately 18 watts. With this energy input, a turbine speed of 22,000 rpm, and using the abbreviations in the attached velocity diagram, the calculated velocities for the Terry turbine 4 R are:
Stage 1, Va1= 1,271 ft/sec, Vb= 86 ft/sec, Vr1= 1,197 ft/sec, Vr2= 546 ft/sec, and Va2= 474 ft/sec. The first stage torque is estimated to be 0.16 in-oz.
Stage 2, Va3= 491 ft/sec, Vb= 86 ft/sec, Vr3= 402 ft/sec, Vr4= 362 ft/sec, and Va4= 291 ft/sec. The second stage torque is estimated to be 0.07 in-oz.
Stage 3, Va5= 326 ft/sec, Vb= 86 ft/sec, Vr5= 233 ft/sec, Vr6= 210 ft/sec, and Va6= 142 ft/sec. The third stage torque is estimated to be 0.04 in-oz.
The velocity drop is so much higher in the first stage since the approaching blades are empty and some of the air from the nozzle suddenly expands without doing any work. Also, when blades are in a position where part of the channel is on the nozzle side and part of the channel is under the next nozzle, the air impacts air moving at a lower velocity. Since the velocity of the air exiting the last stage is not much higher than the blade speed, there is not enough energy left for another stage.
8 March 2019 at 19:29 #399313Turbine Guy
Participant@turbineguyIn the post of 27/02/2019 I stated the following, although not in this order: ‘The blade width (e) should be approximately 3.25 times the nozzle diameter (h). My turbine has a pocket width (e) of 0.125 in. that is approximately 4.46 times the nozzle diameter (h) of 0.028 in. My turbine has an admission length (a) of 0.102 in. and a pitch length (t) of 0.114 in. so the ratio of a/t is approximately 0.89. The average rotor velocity coefficient for partial admission was found to be approximately (1-t/2a) times the maximum rotor velocity coefficient.’ The following drawing shows a concept that makes the following improvements. This turbine has a pocket width (e) of 0.094 in. that is approximately 3.36 times the nozzle diameter (h) of 0.028 in. and very close to the ideal ratio of 3.25. This turbine has an admission length (a) of 0.102 in. and a pitch length (t) of 0.057 in. so the ratio of a/t is approximately 1.79. The factor (1-t/2a) is 0.44 for my existing turbine and is 0.72 for this turbine. Since the pockets are similar, the maximum rotor velocity coefficients should be approximately the same. The average rotor velocity coefficient could be 1.64 times larger for this turbine than for my existing turbine.
 10 March 2019 at 08:51 #399515
10 March 2019 at 08:51 #399515Martin Johnson 1
Participant@martinjohnson1Turbine Guy,
Thank you for these posts they are a useful input to a topic that has defied model engineers for a long time.
I was not aware of the Terry turbine (having been brought up in a company that made big steam turbines with proper fir tree root blades), but the concept looks very good for miniature builds. I cannot quite see how the Terry velocity staging works (despite studying your drawings and the Terry illustration), so more clarity on the casing return passages would help.
It seems to me the fundamental problem in model turbines is that we are dealing with tiny steam flows. Hence, disk friction losses in the turbine are going to be relatively huge. Therefore, whatever means we can use to reduce running speed will all be to the good. I am sure I had an efficiency estimation chart at one time, which showed that as the turbine type number reduces then the efficiency also heads south – due to the disk friction losses.
One method of reducing running speed (and increasing type number) that I saw in an ME, probably from the '50s comprised a steam ejector working into an axial flow turbine. I rather discounted the idea at the time as ejectors are notoriously inefficient. HOWEVER, with further thought it is the momentum input to a turbine that matters and ejectors are very good at changing momentum from a very fast small jet to a slower big jet with THE SAME MOMENTUM. Given that turbocharger rotors can be readily found in scrapyards, I wonder if an ejector / radial inflow turbine combination would give good results.
My own interest is in developing a turbo generator for charging a 12 volt car battery – so quite big.
Martin
10 March 2019 at 17:29 #399614Turbine Guy
Participant@turbineguyMartin,
Thanks for your kind comments.
I may have confused you by using the velocity diagram of an 3 velocity stage axial impulse turbine. I used that diagram for showing the change in velocity as the flow goes through 3 stages. The axial turbine would actually have 3 rows of stationary nozzles and 3 rows of blades on the rotor and the flow would go as shown on the diagram. The principle is the same for the Terry turbine but it only requires 1 row of blades on the rotor and 1 row of stationary blades that act as nozzles in the reversing chamber. The flow issuing from the nozzle passes through the first set of rotor blades and into the first set of blades in the reversing chamber. The flow entering in the first set of blades in the reversing chamber is turned 180 degrees and advanced to where it will enter the next set of blades on the rotor. The flow in the Terry turbine is cork screw like shown below.
The disk friction is high for turbines but much less of a factor for models with their small rotor diameters. The rotational loss is proportional to the cube of the rotor diameter (D^3) so becomes more of a problem as the diameter is increased. The loss in efficiency of the small turbines is primarily caused by not being able to run at higher speeds. Dental drill turbines operate at speeds well over 200,000 rpm.
The steam ejector idea is an interesting concept. I'll see if I can get a rough idea how effective it would be in my next post.
11 March 2019 at 09:37 #399701Martin Johnson 1
Participant@martinjohnson1Hi T.G.,
" I may have confused you by using the velocity diagram of an 3 velocity stage axial impulse turbine. "
Well, I have been constructing and reading velocity diagrams for long enough to realise that what you showed was not what you were talking about. Thanks for the extra explanation on the return passages in the Terry turbine casing. So it seems in practical terms the axis of the cutter for the return passage has to be tilted over from radial (viewed in the axial plane) to achieve the return of steam to the "next" blade in the rotor.
Sorry I can't find you a reference to the ejector – turbine combination, but it was a long time ago. The builder was looking to replace a Stuart Double 10 with a turbine, and was trying to get equivalent power from the same boiler – IIRC!
Martin
11 March 2019 at 16:02 #399746Turbine Guy
Participant@turbineguyHi Martin,
I tried to find a efficient method for using an ejector supplied with high pressure pumping a larger volume at a lower pressure. The pumping efficiency of the ejectors was very low using the data that gave enough information to calculate the efficiency. I looked at using steam and air for the operating medium pumping either air or water. In all cases the amount of input energy required was far more than the energy imparted to the medium being pumped. The injectors have been used to supply feed water to boilers so are capable of pumping water at a higher pressure than the steam in the boiler. A advantage of the injectors used to feed boilers is the losses are converted to heat and warm the feed water so they recover most of the energy lost in pumping.
11 March 2019 at 21:04 #399779duncan webster 1
Participant@duncanwebster1I'm not sure this ejector idea works. The force exerted on the blades is the momentum flow rate * the change in whirl speed (see below). For single stage impulse the change in whirl speed is roughly twice the blade speed (up to the limit of blade speed = half steam speed). The mix of air and steam from the ejector has the same momentum flux as the steam alone, so for a given blade speed the blade force is a constant. However, the power output of the turbine is blade force * blade speed, so the for a given blade speed the power is the same. However, if you can physically achieve it, you can have a much higher blade speed for steam alone, hence higher power.
Newton's second law, usually expressed as F=m*a can equally well be expressed as F = rate of change of momentum
12 March 2019 at 09:40 #399844Martin Johnson 1
Participant@martinjohnson1Since I have remembered the idea of the ejector driven turbine for so long, you can tell it fascinates me.
Duncan, I agree that the momentum flux stays the same but as you say the velocity drops and the mass flow increases. and from Newtons law the force would stay just the same.
HOWEVER, designing model turbines seems to me a battle against bearing problems, disk friction and how to make stupidly small passages / blades. It seems to me that converting the momentum to more mass flow and lower velocity answers all three of those problems. I think we can accept some inefficiency in the ejector because turbine efficiency at very low flows just plummets, so to offset the ejector loss we have a gain in turbine efficiency.
Turbine Guy – Your argument is the one I swallowed for years. But as Duncan says, the momentum flux stays just the same and since turbines actually need momentum flux, then are the losses not small?
One of us is going to have to make one!
Martin
12 March 2019 at 11:28 #399863duncan webster 1
Participant@duncanwebster1Mea culpa, I've got it wrong! If you are stuck with a low blade speed, there are advantages in the ejector idea, but you'd still be better off with steam only and very high speeds
Blade force is mass flow rate * change in whirl velocity, so increasing the mass flow rate for a given blade speed increases the blade force. If for steam only we have a mass flow of M kg/sec and a blade speed of B, the blade force is M * B * 2, and the power output is force * blade speed = M * B^2 * 2
Mixing in an equal mass of air, the mass flow is 2M, for the same blade speed, the blade force is 2M * B * 2, and the power is 2M * B^2 * 2, so there is an advantage.
You're still better off with higher blade speed tho as the change in whirl velocity increases as well.
12 March 2019 at 17:27 #399958Turbine Guy
Participant@turbineguyMartin and Duncan,
You both make some very good points. I'm going to look further into this, taking into account all the mass flow and energy of the combined air and steam. I'll post what I come up with.
13 March 2019 at 04:31 #400063duncan webster 1
Participant@duncanwebster1Martin and Turbine guy, I've pm'd you both
13 March 2019 at 12:24 #400142Turbine Guy
Participant@turbineguyThe following analysis is based on the information given in a Penberthy Jet Pump Technical Data brochure from the following link. **LINK** . This brochure gives enough data to make a reasonable estimate of the combined mass flow and energy of the air and steam. I base the analysis on their smallest jet pump.
The first step in their guidelines for pumping gases with steam is to find the suction capacity (Qs) from the performance curves for the inlet air pressure, the steam pressure, the discharge pressure, and model of jet pump. For an inlet pressure of 0 psig, a steam pressure of 80 psig, a discharge pressure of 21 psig, and a model GH jet pump, the suction capacity is approximately 29 SCFM.
The second step is to find from the Capacity Factor table, the capacity factor for the size of the jet pump. For the smallest jet pump, the capacity factor is 0.030.
The third step is to find from the Steam Consumption table, the steam consumption of a size 1½ model for the steam pressure. For 80 psig steam pressure, the steam consumption is 623 lb/hr for a size 1½ model GH jet pump.
The forth step is to find the steam consumption of the smallest size GH jet pump. The steam consumption for the smallest GH jet pump is found by multiplying the steam consumption of the 1½ size by the Capacity Factor. 623 x 0.030 = 18.7 lbs/hr.
The fifth step is finding the amount of air pumped by the smallest GH jet pump. The volume of air pumped for the smallest GH jet pump is found by multiplying the volume of air pumped of the 1½ size by the Capacity Factor. 29 x 0.030 = 0.87 SCFM. For 0.87 SCFM, the mass flow of air is 3.9 lb/hr. The inlet conditions of the air are, the pressure is 0 psig, the temperature is 70 F, the specific volume is 13.3 ft^3/lb, and the enthalpy is 126.7 btu/lb.
For 80 psig saturated steam, the temperature is 324 F, the specific volume is 4.7 ft^3/lb, and the enthalpy is 1186.2 btu/lb. The conditions at the exit of the steam nozzle are the pressure is 21 psig, the temperature is 261 F, the specific volume is 10.9 ft^3/lb, and the enthalpy is 1111.0 btu/lb. The enthalpy drop across the nozzle is 75.2 btu/lb and the velocity energy in is 75.2 btu/lb x 18.7 lb/hr = 1406 btu/hr.
Assuming the impact energy and the heat in the steam raises the air temperature to 280 F and the pressure to 21 psig, the enthalpy of the air is 164 btu/lb and the enthalpy increase in the air is 164 – 126.7 = 37.3 btu/lb. The energy increase is 37.3 btu/lb x 3.9 lb/hr = 145 btu/hr. Since the increase in energy of the air is only a fraction of the velocity energy, the remaining energy after impact must raise the temperature of the steam. The amount of velocity energy that reheats the steam is 1406 btu/hr – 145 btu/hr = 1261 btu/hr. The enthalpy increase in the steam is 1261 btu/hr / 18.7 lb/hr = 67.4 btu/lb. For a enthalpy increase of 67.4 btu/lb, the steam temperature raises to approximately 280 F.
13 March 2019 at 12:28 #400143Turbine Guy
Participant@turbineguyThe discharge conditions for the mixture are the pressure is 21 psig, the temperature is 280 F, the enthalpy is 1186 btu/lb, and the mass flow is 22.6 lb/hr. The isentropic enthalpy drop to a pressure of 0 psig is 1186 – 1111 = 75 btu/lb. The exit velocity from a nozzle with this enthalpy drop is 1,938 ft/sec. The energy of the mixture is 75 btu/lb x 22.6 lb/hr = 1695 btu/hr
The isentropic enthalpy drop from the 80 psig and 324 F to a pressure of 0 psig is 1186 – 1048 = 138 btu/lbm. The exit velocity from a nozzle with this enthalpy drop is 2,629 ft/sec. The energy of the steam is 138 btu/lb x 18.7 lb/hr = 2581 btu/hr.
The nozzle velocity is reduced but at the expense of a large loss in available energy.
13 March 2019 at 16:53 #400193Martin Johnson 1
Participant@martinjohnson1Thanks for making me think, chaps.
You are right for constant momentum, but greater mass flow, the TORQUE would remain constant for a given design of blades, but at the same time the optimum running speed would fall in the ratio of the mass flow increase (same as velocity decrease). Hence power falls as the ratio of mass flow increase or velocity decrease. All assuming motive and entrained fluids of identical density.
BUT earlier in this thread TG is reporting a blade speed to fluid speed ratio of 0.022 – way down on where it should be for a two velocity staged machine. AND even if he can get the running speed up to where it should be, how long will the bearings stand up to it? Or, indeed, how long will the blades stay attached to the hub? AND what losses would there be in a wee gearbox to reduce the speed to something manageable?
So, the question remains for tiny turbines, would an ejector lose more than it gains?
For all that, I am in awe of the tiny rotor and blade cutting that TG is undertaking. Also, the methodical way it is being thought through. Keep the reports on progress coming, please.
Martin
13 March 2019 at 18:54 #400218Turbine Guy
Participant@turbineguyThe following is a similar analysis as in the 13/03/2019 posts for the GH ½ B model jet pump
Air inlet pressure of 0 psig
Air inlet temperature of 70 F
Air inlet enthalpy of 126.7 btu/lb
Steam pressure of 120 psig,
Steam temperature is 351 F
Steam enthalpy is 1192 btu/lb.
Suction capacity is approximately 54 SCFM.
½ B Capacity factor is 0.030.
1½ model steam consumption is 390 lb/hr
½ B model steam consumption is 11.7 lb/hr
½ B air pumped = 1.6 SCFM
½ B mass flow of air is 7.2 lb/hr
Discharge pressure is 12 psig,
Discharge temperature is 300 F
Discharge enthalpy is 1192 btu/lb
Discharge mass flow is 18.9 lb/hr.
The mixture isentropic enthalpy drop to a pressure of 0 psig is 1192 – 1144 = 48 btu/lb. The exit velocity from a nozzle with this enthalpy drop is 1550 ft/sec. The energy of the mixture is 48 btu/lb x 18.9 lb/hr = 907 btu/hr
The isentropic enthalpy drop from the 120 psig and 351 F to a pressure of 0 psig is 1192 – 1045 = 147 btu/lbm. The exit velocity from a nozzle with this enthalpy drop is 2,713 ft/sec. The energy of the steam is 147 btu/lb x 11.7 lb/hr = 1720 btu/hr.
This model jet pump was designed to move higher flows with less steam and works better. The extra mass flow and lower velocity increases the nozzle size or quantity which can make a substantial difference on small turbines.
I'll read the discussions on momentum exchange and respond.
Edited By Turbine Guy on 13/03/2019 19:00:55
13 March 2019 at 23:13 #400257Turbine Guy
Participant@turbineguyMartin,
The equation I use for the power of an ideal impulse turbine might help show the effect of changes.
P =2MW(V-W)/g
Where
P is the power
M is the mass flow
W is the rotor speed at the blade location
g is the acceleration of gravity
V is the spouting velocity of the nozzleIncreasing the mass flow or spouting velocity without any other changes will always increase the power. Increasing the rotor speed up to a maximum of ½ the spouting velocity will also increase power in the ideal case but rotational losses have to be dealt with in real turbines.
What I found in the last post is starting to convince me using an ejector might actually help. I'm going to look at this a little more.
I appreciate you comments on my methodical approach and machining the tiny blades. I enjoy the engineering and design as much as making the models. Because of the cost of the tools required and my limited machining skills, It will be a while before I try making the model Terry turbine. I have an improved version of the open pocket rotor I plan to make next.
14 March 2019 at 18:14 #400383Turbine Guy
Participant@turbineguyI finally have enough information to give an indication of how effective using an ejector could be for small turbines. A Penberthy GL ½ A ejector has the following performance.
Air inlet pressure of 0 psig
Air inlet temperature of 70 F
Steam pressure of 80 psig,
Steam temperature of 324 F
Steam enthalpy of 1186 btu/lb
Steam consumption of 8.3 lb/hr
Amount of air pumped = 1.77 SCFM
Mass flow of air is 8.0 lb/hr
Discharge pressure is 6 psig,
Discharge temperature is 290 F
Discharge specific volume is 21 ft^3/lb
Discharge enthalpy at 6 psig is 1186 btu/lb
Discharge enthalpy at 0 psig is 1154 btu/lb
Discharge mass flow is 16.3 lb/hr.
Discharge isentropic enthalpy drop to a pressure of 0 psig of 32 btu/lb.
Steam enthalpy at 0 psig is 1047 btu/lb
Steam isentropic enthalpy drop to a pressure of 0 psig is 139 btu/lb.
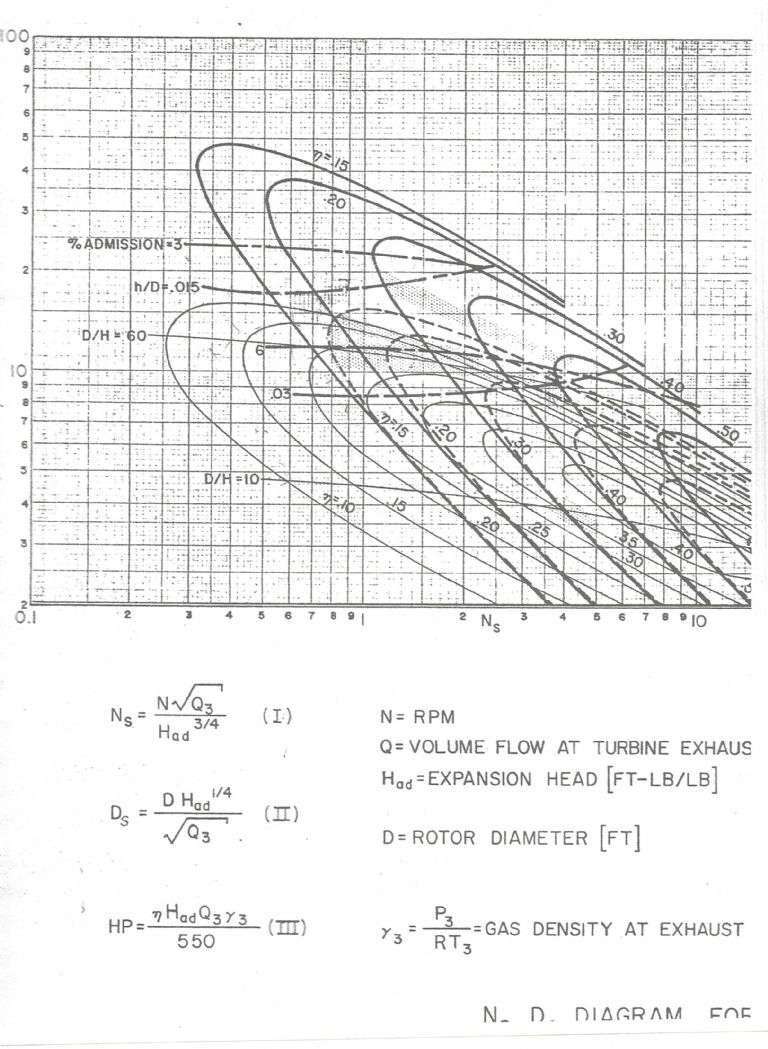
Steam specific volume at 0 psig of 24 ft^3/lbWith this information I can use the following chart to estimate the performance with and without the ejector. This chart estimates the maximum performance for the given specific speed Ns and specific diameter Ds. It is intended to be used by first deciding the speed you would like to run and then calculating the specific speed Ns. You then use Ns to find the Ds that gives the best efficiency. You can then find the optimum rotor diameter from the Ds. I am using a turbine speed of 10,000 rpm for this comparison.
For the ejector, Q3 = 0.095 ft^3/sec, Had = 24,896 ft-lb/lb, and Ns = 1.56
From the chart for Ns = 1.56 the maximum efficiency is approximately 34% with Ds =18
For Ds = 18, the rotor diameter is 5.30 in. The power is 0.070 hp.Without the ejector, Q3 = 0.055 ft^3/sec, Had = 108,142 ft-lb/lb, and Ns = 0.39
From the chart for Ns = 0.39 the maximum efficiency is approximately 17% with Ds =40
For Ds = 40, the rotor diameter is 6.21 in. The power is 0.076 hp.Using the ejector, the efficiency of the turbine is double that of the turbine without the ejector. The ejector definitely improves the transfer of energy in the turbine. However, the discharge mixture from the ejector has so much less energy that the power ends up almost the same.
 15 March 2019 at 12:05 #400520
15 March 2019 at 12:05 #400520Turbine Guy
Participant@turbineguyThe heavy solid lines in the chart of the preceding post are for axial turbines. The dashed lines are for Terry turbines. The thin solid lines are for Drag turbines. The drag turbines are like turbine pumps. The blades circulate the flow in a way that increases the drag force on the rotor. The units for the volume flow are ft*3/sec and for the gas density lb/ft^3.
Edited By Turbine Guy on 15/03/2019 12:08:55
26 March 2019 at 19:13 #402324Turbine Guy
Participant@turbineguyIn the post of 08/03/2019 I showed a revised rotor and stated that it could get a rotor velocity coefficient of up to 1.64 times that of my existing turbine rotor. I built this rotor almost as shown on the drawing attached to the 08/03/2019 post. The following photo shows the new rotor (aluminum) next to the original rotor (brass) I tested this rotor with the same air pressure and flow used in the test of my original rotor that had a maximum speed of 17,000 rpm. The maximum speed with the new rotor was 18,250 rpm. The required power of the EP2508 propeller used in these tests is approximately 1.9 watts at 17,000 rpm and 2.4 watts at 18,250 rpm. The average rotor velocity coefficients for these output powers are 0.34 for 1.9 watts and 0.53 for 2.4 watts. The increase in rotor velocity coefficient with the new rotor is 1.56 times. I didn't quite get the maximum I thought was possible, but this is a very significant increase. The original rotor has 24 pockets and the new rotor has 48 pockets. In Dr. Balje's study of high energy level, low output turbines the highest average rotor velocity coefficient for a Terry turbine with a single nozzle and 45 blades was 0.53 The open pockets appear to be as efficient as the Terry turbine blades.
10 April 2019 at 21:06 #404521Turbine Guy
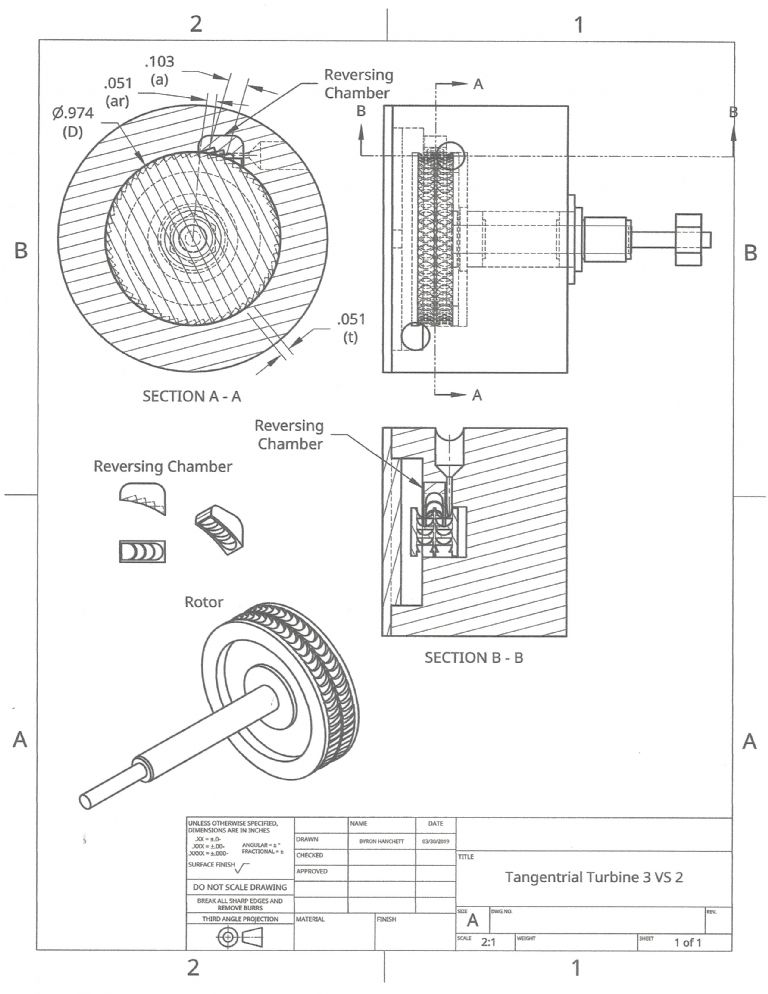
Participant@turbineguyBased on the success of the new rotor that verified the importance of using as many blades or pockets as practical, I’m going to try velocity staging next. I looked at making a Terry turbine with a reversing chamber as shown on the drawing of the 04/03/2019 post. I was able to come up with ways to machine the blades but since the performance of the open pockets appeared to be about the same as with blades, I’m looking at other possibilities. I compared the estimated performance of the Terry turbine with an open pocket design that used two rows of blades on the rotor and an open pocket reversing chamber as shown on the following drawing. Since a new housing is needed, I increased the number of pockets from 48 to 60. I also made the ratio’s of the nozzle admission length (a), reversing chamber admission length (ar), and the rotor pitch length (t) approximately even multiples (a/t = 0.103/.051= 2.02 and ar/t = 0.051/0.051 = 1). With these improvements to the open pocket design, the estimated maximum power was about the same as for the Terry design. The wider blades required for the corkscrew type of flow of the Terry turbine resulted in about 2.5 times the flow length of the open pocket design per stage. Both types allow the flow to expand as it travels, so the length of travel is very important. Dr. Balje’s methods of estimating performance take this into account and were what I used to in my comparison.
10 April 2019 at 21:29 #404531DrDave
Participant@drdaveI was wondering how you were going to machine the reversing chamber around the whole internal circumference: I had forgotten that you only need a short length. A very elegant design! I hope that it runs well.
13 April 2019 at 09:58 #404862Martin Johnson 1
Participant@martinjohnson1Turbine Guy,
I have just caught up with your post of 14/3/19 doing outline design calculations for a turbine and a turbine / ejector combination. Carefully thought through again, and interesting that it shows things to be roughly the same – i.e. ejector losses balance turbine efficiency gains. Thanks for that.
How well do you think the Ds Vs. Ns chart reflects things in your tiny turbines?
I will be very interested to see what you get from the two stage open pocket design. – I dare say you will be as well.
Thanks for a great thread,
Martin
-
AuthorPosts
- Please log in to reply to this topic. Registering is free and easy using the links on the menu at the top of this page.
Latest Replies
Home › Forums › Stationary engines › Topics
-
- Topic
- Voices
- Posts
- Last Post
-
-
Noisey Gears?
Started by:
Martyn Nutland 1 in: Workshop Tools and Tooling
- 11
- 12
-
19 April 2025 at 02:05
 peak4
peak4
-
‘150 Case’ road locomotive pulling 44 ploughs.
Started by:
Ches Green UK in: Traction engines
- 3
- 3
-
19 April 2025 at 00:47
howardb
-
Stopping milling chips going everyehere
Started by:
petro1head in: General Questions
- 11
- 13
-
19 April 2025 at 00:03
petro1head
-
Change Wheels
Started by:
Julian Goodyear in: Workshop Tools and Tooling
- 4
- 12
-
18 April 2025 at 23:24
Julian Goodyear
-
Solar lights battery AA 900mAh 1.2V
Started by:
JimmieS in: The Tea Room
- 4
- 7
-
18 April 2025 at 22:52
Howard Lewis
-
VFD Article in May issue 351
Started by:
 Robert Atkinson 2
in: Model Engineer & Workshop
Robert Atkinson 2
in: Model Engineer & Workshop
- 1
- 1
-
18 April 2025 at 22:34
 Robert Atkinson 2
Robert Atkinson 2
-
Arduino controlled stepper motor for Mill X-axis drive
Started by:
nevillet in: CNC machines, Home builds, Conversions, ELS, automation, software, etc tools
- 12
- 25
-
18 April 2025 at 22:31
SillyOldDuffer
-
Motor bearings and more
Started by:
 Sonic Escape
in: General Questions
Sonic Escape
in: General Questions
- 6
- 18
-
18 April 2025 at 22:24
 Sonic Escape
Sonic Escape
-
SeaCURE
Started by:
 Michael Gilligan
in: The Tea Room
Michael Gilligan
in: The Tea Room
- 2
- 2
-
18 April 2025 at 20:03
 Thor 🇳🇴
Thor 🇳🇴
-
Acceptable feed screw backlash
Started by:
david newman 9 in: Hints And Tips for model engineers
- 11
- 12
-
18 April 2025 at 18:53
Speedy Builder5
-
Stuart Twin Victoria (Princess Royal) Mill Engine
1
2
…
48
49
Started by:
Dr_GMJN in: Work In Progress and completed items
- 32
- 1,221
-
18 April 2025 at 18:26
 JasonB
JasonB
-
Air source heat pumps
1
2
3
Started by:
Plasma in: The Tea Room
- 27
- 63
-
18 April 2025 at 17:40
duncan webster 1
-
Lercanidipine
Started by:
 Michael Gilligan
in: The Tea Room
Michael Gilligan
in: The Tea Room
- 3
- 4
-
18 April 2025 at 16:27
old mart
-
Gear cutter numbers
Started by:
 Bazyle
in: Workshop Tools and Tooling
Bazyle
in: Workshop Tools and Tooling
- 2
- 2
-
18 April 2025 at 15:50
DC31k
-
Newbie from the North West
Started by:
leakygasket in: Introduce Yourself – New members start here!
- 5
- 6
-
18 April 2025 at 15:08
leakygasket
-
Colchester Chipmaster tailstock shimming
Started by:
Peter_H in: Manual machine tools
- 11
- 18
-
18 April 2025 at 13:29
 Bazyle
Bazyle
-
50,000 Ton Press
Started by:
 Vic
in: The Tea Room
Vic
in: The Tea Room
- 7
- 10
-
18 April 2025 at 11:16
Kiwi Bloke
-
Source of 8mm Dia flexible stainless steel tubing
Started by:
Greensands in: Materials
- 12
- 19
-
18 April 2025 at 11:11
Kiwi Bloke
-
Shop Tips
Started by:
 Vic
in: The Tea Room
Vic
in: The Tea Room
- 6
- 9
-
17 April 2025 at 22:31
 Sonic Escape
Sonic Escape
-
Wheel and track standards
Started by:
 Russell Eberhardt
in: Locomotives
Russell Eberhardt
in: Locomotives
- 2
- 3
-
17 April 2025 at 21:37
Speedy Builder5
-
Mounting chuck directly to rotary table.
Started by:
old mart in: Hints And Tips for model engineers
- 5
- 7
-
17 April 2025 at 19:20
old mart
-
Building Bernard Tekippe’s Precision Regulator
Started by:
Chris Raynerd 2 in: Clocks and Scientific Instruments
- 8
- 21
-
17 April 2025 at 18:06
John Haine
-
Not a dial indicator!
Started by:
 Sonic Escape
in: The Tea Room
Sonic Escape
in: The Tea Room
- 6
- 8
-
17 April 2025 at 17:15
Gerard O’Toole
-
ME No155 Vol10 pg 359 of 1904
Started by:
 JasonB
in: Stationary engines
JasonB
in: Stationary engines
- 7
- 16
-
17 April 2025 at 16:06
 JasonB
JasonB
-
Hitachi SJ300 VFD problem
Started by:
Clive Steer in: Electronics in the Workshop
- 3
- 3
-
17 April 2025 at 14:24
Peter Cook 6
-
Noisey Gears?
-
Latest Issue
Newsletter Sign-up
Latest Replies