Please bear with me … this is rather convoluted, but I think it raises some interesting issues which are quite widely relevant.
I have an amateur interest in micro-manipulation, both manual and mechanical. What these techniques have in common is that they both require appropriately microscopic tools. Useful tools range from natural spines &hairs to items crafted from metal or glass.
On my ‘project list’ is a glass-working station for making the tools.
These can be heated by gas, electricity, or both. … but for physical contact with molten glass the use of a heated Platinum wire is preferred, because it is not wetted by the glass.
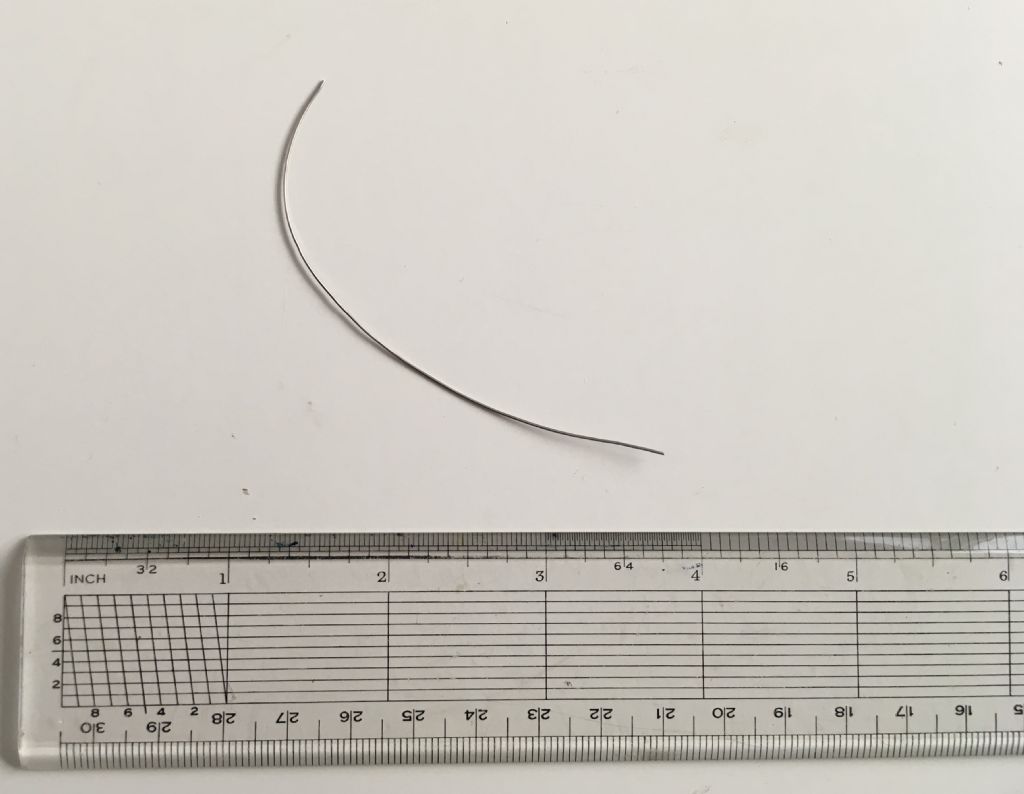
So … I have been on the lookout for Platinum wire, and recently purchased this piece:

.
It was described as 99.99% pure Platinum with approximate dimensions of 0.5mm diameter and 100mm length, and an approximate weight of 0.4g
For no particular reason I felt, and still feel, confident that the Seller was honest.
When it arrived, however, I decided to just check that the numbers tallied
The density of pure Platinum is quoted as 21.45 g/cc and therefore my piece should weigh around 0.42g
The Mitutoyo digital calliper suggested some variation between 0.49 and 0.51 diameter,but the wire will never be straight enough for it to be worth using the micrometer.
The length was simply checked against the plastic rule and is near-enough 100mm [it has been cut with nippers, and seeking better accuracy is probably futile]
I then weighed it on my good, but uncalibrated, scale at 0.40g
… which is also happens to be what the seller’s photo of his weighing showed.
All of this together suggests that the material is basically Platinum, but it goes nowhere near proving that it is 99.99% pure.
Using the nominal dimensions the weight is about 5% too low.
The questions that I invite you to ponder are:
What is the overall Uncertainty of Measurement in what I have done ?
and
Given that there is no traceable certification of purity for this piece, is there any affordable way of checking it more accurately.
Please note: I don’t expect any numerical answer to the first question, it is essentially rhetorical. …I just want you to think about the way we put trust in the numbers our instruments display, with little but blind faith.
MichaelG.
Bruce Voelkerding.